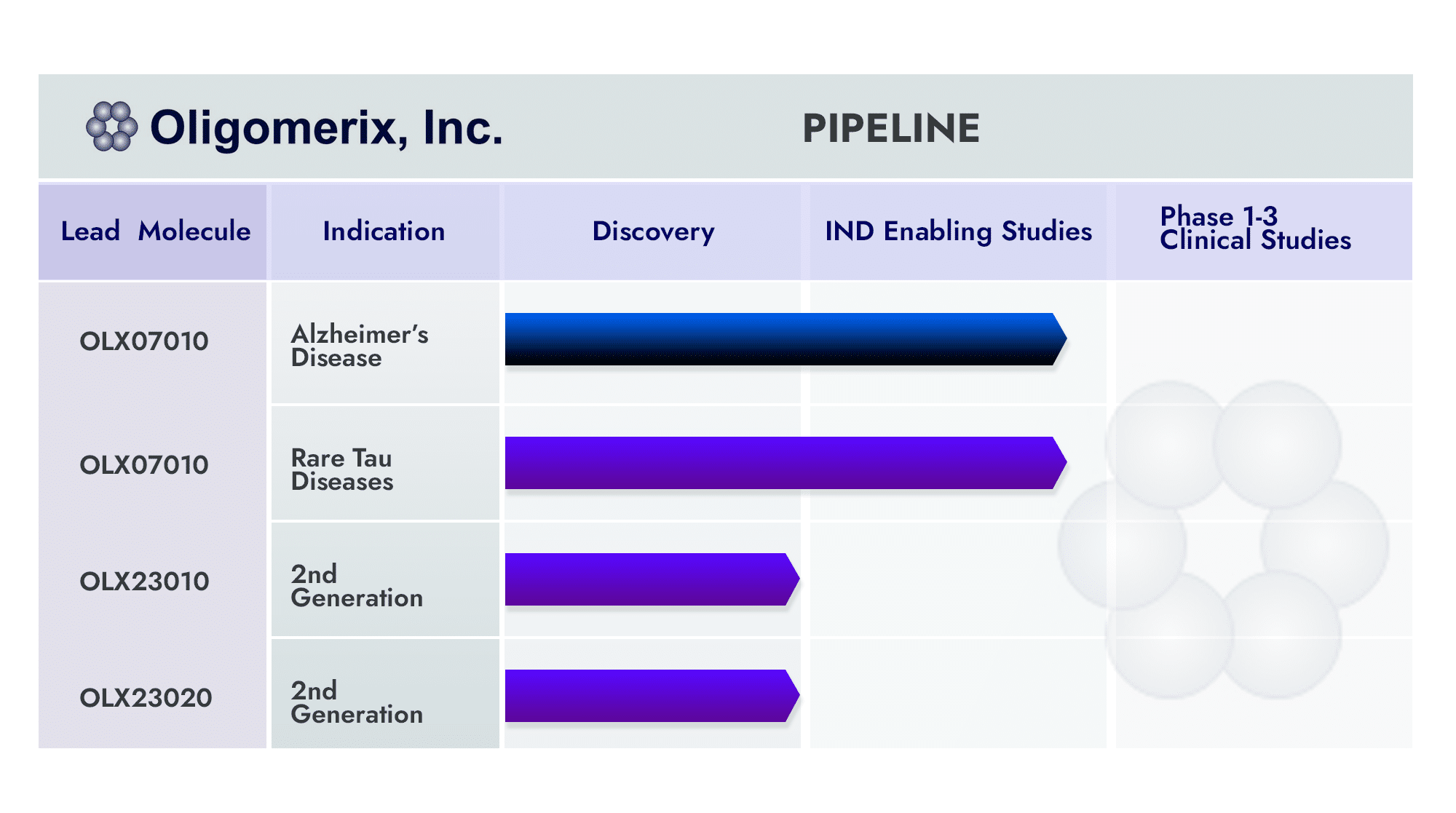
The Alzheimer’s clinical lead program is for the development of a small molecule inhibitor of tau self-association OLX-07010 which has demonstrated proof of concept across multiple transgenic mouse models of tauopathy. Treatment of young mice was effective in inhibiting the formation of tau aggregates in human tau mice modeling tau aggregation in Alzheimer’s disease (Davidowitz EJ et al., 2020) and in mice modeling tau aggregation in inherited forms of PSP or FTD in which mutated tau is more prone to misfold and aggregate (Davidowitz EJ et al., 2023).The activity in these two models translated from in vitro and cellular assays to an in vivo model of tau aggregation validating our screening approach and showing that targeting tau-self association can inhibit the entire tau aggregation pathway.
Moreover, acute therapeutic treatment of aged mice that had already developed tau aggregates inhibited the progression of tau aggregation (Patel DR et al., 2024). OLX-07010 was also evaluated in transgenic mice designed to develop both tau and amyloid beta aggregates that are hallmarks of pathology in Alzheimer’s disease. Treatment was also effective in inhibiting tau aggregation in this model (Patel DR et al., 2024).
As an oral, small molecule targeting tau-self association (the beginning stages of the tau aggregation cascade), OLX-07010 has the potential to be easily administered to improve patient compliance, lower patient burden based on likely enhanced safety profile compared with recently approved antibody treatments, accessibility on a global scale and manufactured at lower costs compared to other approaches. Additionally, OLX-07010 could complement both tau and beta-amyloid antibody treatment.