Oligomerix Mission:
To discover and develop novel and differentiated small molecules and biomarkers focused on tau oligomer inhibition for the disease-modifying treatment of neurodegeneration and dementias including progressive supranuclear palsy (PSP), frontotemporal dementia (FTD) amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease (AD).
Company Overview:
Oligomerix is a biotech company that has developed a proprietary platform technology for drug discovery and biomarker development for central nervous system (CNS) disorders focused on neurodegeneration and dementias, and has discovered unique, highly differentiated drug-like small molecules that inhibit tau self-association into toxic oligomers.
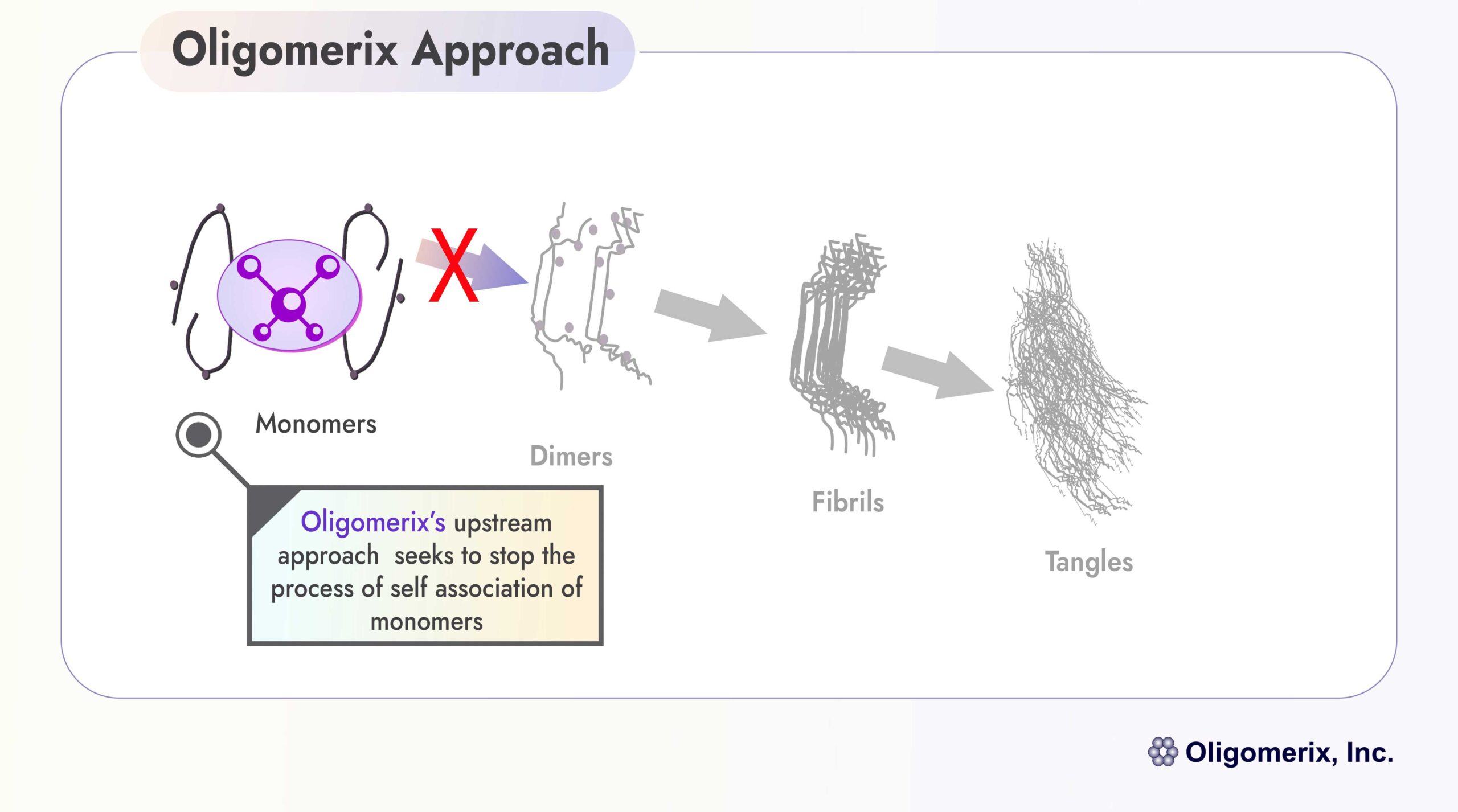
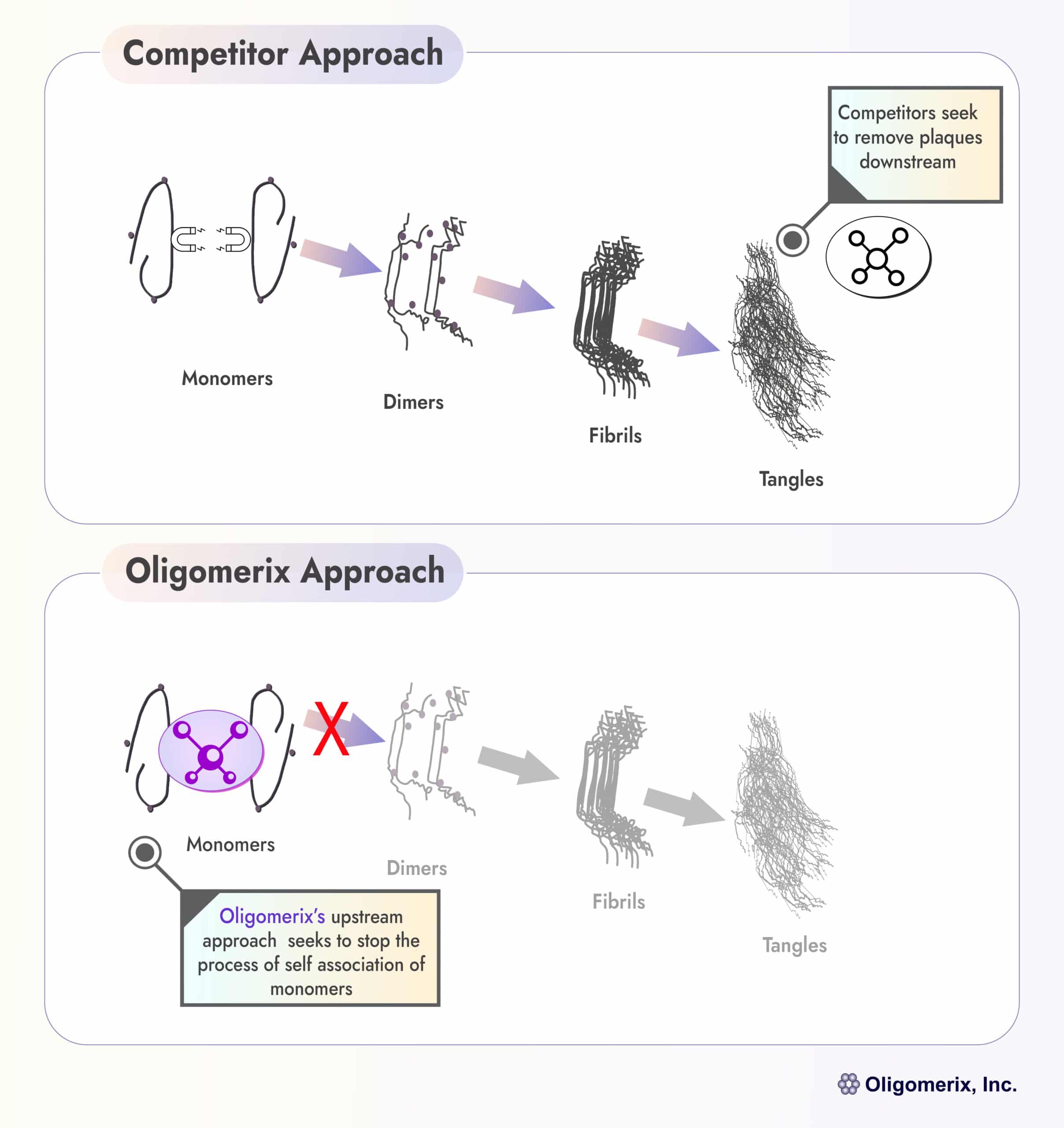
Oligomerix targets the beginning of the tau protein aggregation cascade to minimize the formation of toxic aggregates and downstream tangle formation - thereby preventing neurodegenerative disease progression.
An effective inhibitor of tau self-association, the first step in aggregation in the formation of all pathological multimeric tau structures, has potential utility in the treatment of various tauopathies including PSP, FTD and AD.
Oligomerix has positioned itself to be the premier provider of highly differentiated small molecule therapeutic interventions for blocking the formation of the tau oligomer target.
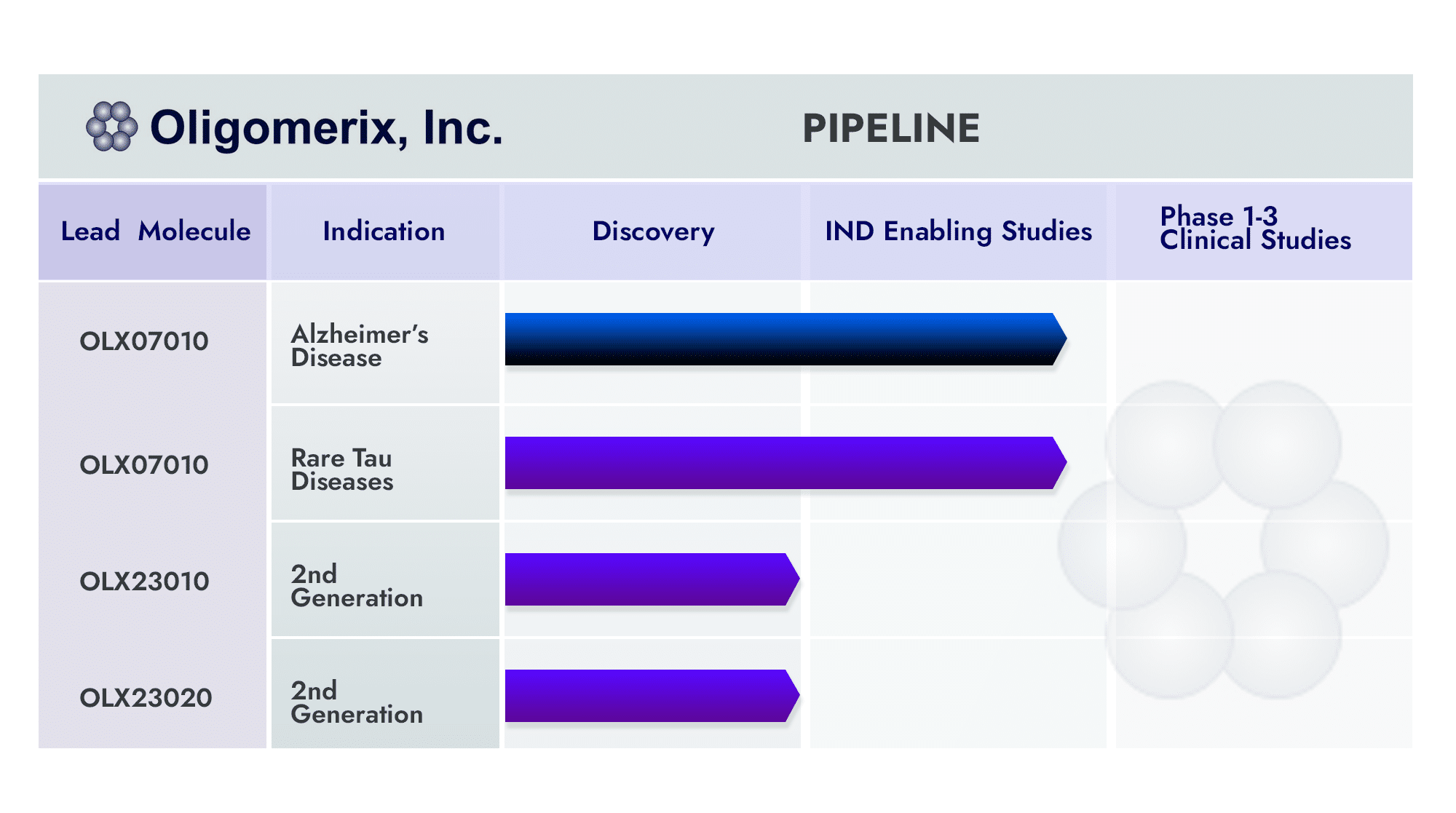
The Company has developed a platform of assays, tools, lead compound (OLX 07010) and 2nd generation series, and know-how that will enable its strategic partner/acquirer to move rapidly into development and clinical evaluation, regulatory submission and launch. The hope is to make available treatment options to neurodegenerative disease sufferers and their caregivers for these devastating diseases.
The Need:
There exists a major unmet medical need in the U.S for disease-modifying therapeutics (DMT) for the treatment of dementia in Rare Diseases such as progressive supranuclear palsy (PSP), frontotemporal dementia (FTD), ALS and the more common Alzheimer’s disease (AD). This unmet need continues to grow given both the increasing prevalence and significantly increasing caregiver costs driven by the aging population. For example, death due to AD remains the 6th leading cause of death in the U.S., increasing 145% since 2000, and remains as one of the costliest diseases in the U.S.
Through our approach, Oligomerix is working towards meeting the global need for effective therapeutic strategies. Our oral, small molecule lead program has several potential advantages including:
- Ease of administration to improve patient compliance
- Lower cost of manufacturing for lower cost of therapy
- Increased patient accessibility on a global scale
- Improved brain penetration
- Oligomerix’s oral small molecule formulation is a critical competitive advantage because it enables cost-effective, patient-centered treatment for the rapidly growing, unserved AD market in the context of significant and growing global healthcare system cost pressures.
Our platform program is progressing to fill this need with a small molecule tau protein targeted DMT and associated biomarker. According to the World Health Organization, there are around 50 million people who suffer from dementia, and almost 10 million new cases reported every year.
While AD is the most common form of dementia, rare neurodegenerative diseases such as PSP and FTD are still significant healthcare and economic problems. Roughly 20,000 people in the U.S. suffer from PSP, while FTD affects 50,000 to 60,000 Americans.
History & Mission Statement:
Oligomerix has progressed its platform tau program including its lead tau small molecule inhibitor of tau self-association to the cusp of IND filing and Phase 1 clinical development.
This achievement is the result of a sole focus on tau protein, enhancing our understanding of the role that tau plays in AD and numerous other tauopathies such as PSP, FTD, ALS and in segments of Parkinson’s disease (LRRK2). The Company has discovered unique, drug-like small molecules that inhibit tau self-association into toxic oligomers. Importantly, this activity occurs at the beginning of the now well characterized tau cascade whereby tau aggregation contributes to the progression of disease in AD and many other tauopathies.
The platform also includes a 2nd generation series of small molecules and companion biomarkers programs. Oligomerix has advanced its technologies using its equity investment (seed through Series B financing and convertible notes), NIH grant awards, a grant from the Alzheimer’s Drug Discovery Foundation (ADDF), and tax credits from New York State (NYS) and New York City (NYC). The NIH has been a leading supporter since the Company was founded via its SBIR grant support.
The Company has built a sound business model around its core technologies, a strong intellectual property (IP) position and a very highly experienced management team.
Oligomerix’s Value Proposition:
The Company’s validated small molecule lead compounds and companion biomarker assays will enable strategic partners to accelerate and de-risk clinical development in the pursuit of disease modifying treatment (DMT) for AD and related tauopathies. The Company’s highly differentiated tau oligomer inhibitor program includes highly valuable assets such as its proprietary screening assays targeting tau self-association that use full length, non-mutated tau target as found in AD, its CNS drug-like leads, and its translatable mechanistic biomarker.
Differentiation: The program is highly differentiated from competitors by targeting tau self-association at the beginning of the tau aggregation cascade that ultimately leads to formation of tau tangles, a hallmark of tau dementias.
Compared to other therapies currently available or in development, our lead program OLX-07010 offers several potential advantages including increased brain penetration, lower cost of manufacturing, ease of oral administration for patient compliance and better patient accessibility on a global scale. Furthermore, OLX-07010 could complement both tau and beta-amyloid antibody treatment.