Oligomerix is in discussions with several potential strategic partners, leading foundations, dementia funds and investors and intends to collaborate in the future on its clinical development program. We welcome the opportunity for further discussions with interested parties that can reach out to us via our contact information page.
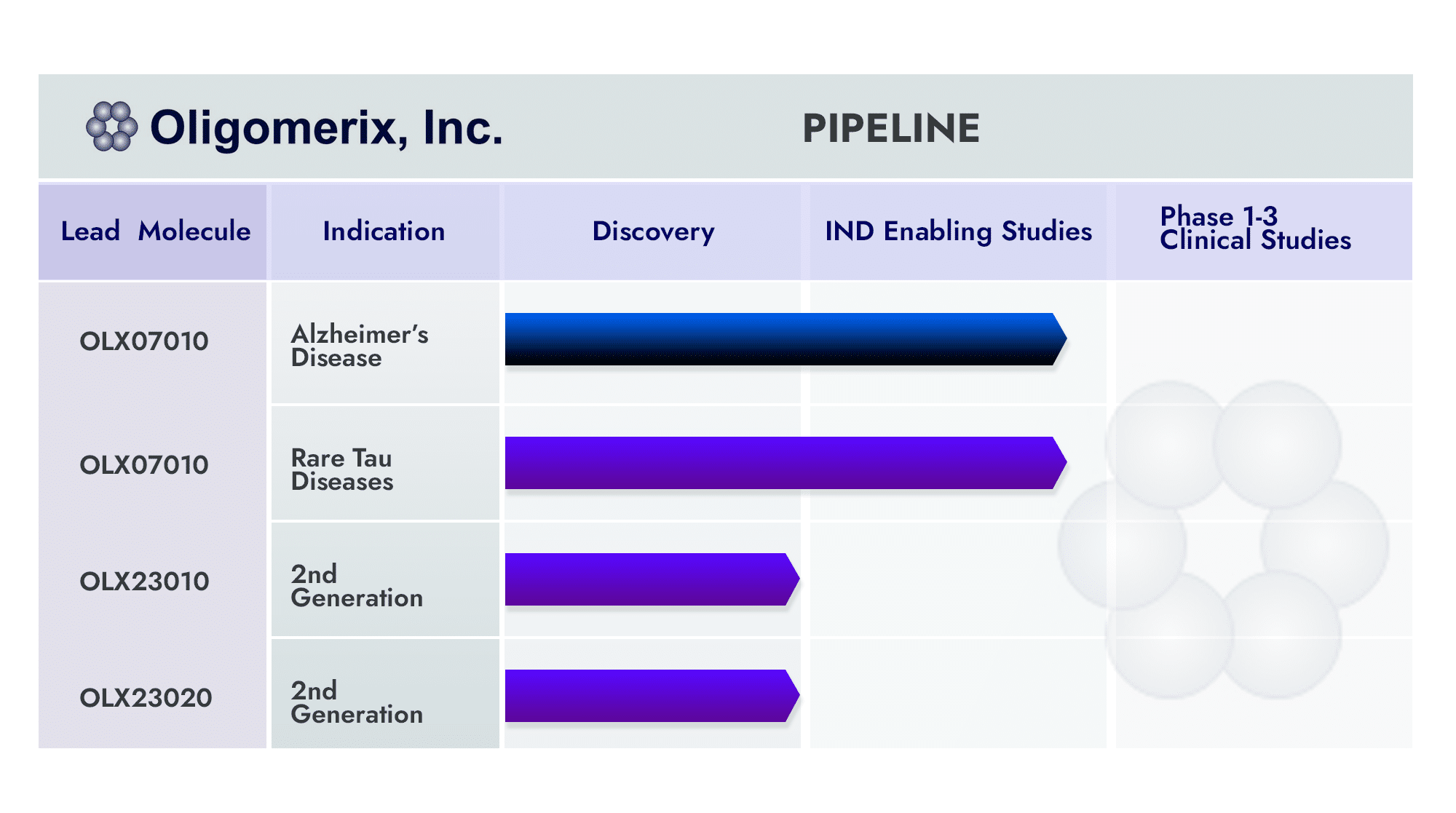
Oligomerix has advanced its OLX-07010 tau self-association inhibitor into first-in-human phase 1a clinical trial in early 2023. This achievement is the result of a sole focus on the tau target for more than 16 years, enhancing our understanding of the role that tau plays in AD and other tauopathies such as PSP.
Through our differentiated approach utilizing an oral, small molecule targeting tau-self association, OLX-07010 could complement both tau and beta-amyloid antibody treatment.